PARAFLUOROANILINE
Chemical properties:
- CAS number: 371-40-4
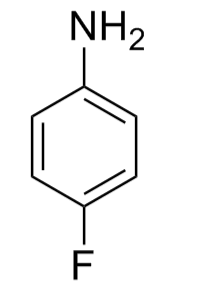
- Parafluoroaniline, also known as 4-fluoroaniline, has the chemical formula
C6H6FN and a molar mass of 111.12 g/mol.
- It is a white to off-white crystalline solid with a melting point of 70-74°C and a
boiling point of 219°C.
- Parafluoroaniline is slightly soluble in water (0.37 g/100 mL at 20°C) and is
soluble in organic solvents such as ethanol, acetone, and benzene.
- It has a density of 1.216 g/cm³ and a vapor pressure of 0.59 mmHg at 25°C.
- Parafluoroaniline is a weakly basic compound, with a pKa of 4.83.
Applications:
-
Parafluoroaniline is primarily used as an intermediate for the synthesis of
pharmaceuticals, agrochemicals, and dyes.
It is a versatile starting material for the preparation of a wide range of organic compounds. - In the pharmaceutical industry, parafluoroaniline is used to produce drugs
such as antipsychotics and anti-inflammatory
agents. - In the agrochemical industry, it is used as an intermediate in the production of herbicides and fungicides.
- In the dye industry, parafluoroaniline is used as a precursor for the synthesis of fluorescent dyes and pigments.
- Parafluoroaniline is also used as a reagent in organic synthesis and as a standard in analytical chemistry.
Safety information for handling, packaging and storage:
-
Parafluoroaniline is considered to be harmful if ingested, inhaled, or absorbed
through the skin. It may cause irritation to the
eyes, skin, and respiratory system. - Long-term exposure to parafluoroaniline may cause damage to the liver,
kidneys, and nervous system. It may also cause
cancer in laboratory animals. - Users should take appropriate precautions, including wearing protective
clothing and gloves, and should avoid prolonged
or repeated exposure to the substance. - In case of skin contact, the affected area should be washed thoroughly with
soap and water. In case of eye contact,
the eyes should be flushed with water for at least 15 minutes. If ingested, medical attention should be sought immediately. - Parafluoroaniline should be stored in a cool, dry place away from sources of
heat and ignition. It should be kept in a tightly
closed container to prevent exposure to air and moisture. - It should be handled with care to avoid exposure. Users should wear
appropriate personal protective equipment, including
gloves, eye protection, and a respirator if necessary. - Spills or leaks should be cleaned up promptly using appropriate methods, and contaminated materials should be disposed of in accordance with local regulations.