PARAFLUOROPHENOL
Chemical properties:
- CAS number: 371-41-5
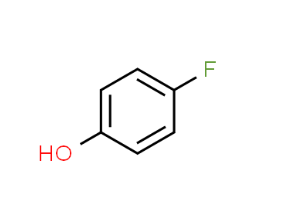
- Parafluorophenol, also known as 4-fluorophenol or p-fluorophenol, has the
chemical formula C6H5FO and a molar mass of 112.1 g/mol.
- It is a white crystalline solid with a melting point of 44-46°C and a boiling point
of 183°C at 760 mmHg.
- Parafluorophenol is sparingly soluble in water but soluble in organic solvents
such as ethanol, ether, and benzene.
Applications:
- Parafluorophenol is commonly used as a building block for the synthesis of various organic compounds, such as pharmaceuticals, agrochemicals, and dyes.
- It is also used as a catalyst in some organic reactions, particularly in the production of resins and polymers.
- In the pharmaceutical industry, it is used as a starting material for the production of drugs such as analgesics and antipsychotics.
- In the agrochemical industry, it is used as a starting material for the production of herbicides and fungicides.
Safety information for handling, packaging and storage:
-
Parafluorophenol is considered to be relatively safe when handled and used
appropriately. It is not known to be carcinogenic
or mutagenic, and there are no known harmful effects on human health from exposure. - However, as with all chemicals, appropriate precautions should be taken
when handling parafluorophenol. Users should wear appropriate personal
protective equipment, including gloves and eye protection, and should avoid
prolonged or repeated
exposure to the substance. - In case of skin contact, the affected area should be washed thoroughly with
soap and water. In case of eye contact, the eyes
should be flushed with waterfor at least 15 minutes. If ingested, medical attention should be sought immediately. - In case of skin contact, the affected area should be washed thoroughly with
soap and water. In case of eye contact,
the eyes should be flushed with water for at least 15 minutes. If ingested, medical attention should be sought immediately. - Parafluorophenol should be stored in a cool, dry place away from sources of
heat and ignition. It should be kept in a tightly
closed container to prevent exposure to air and moisture. - It should be handled with care to avoid exposure. Users should wear
appropriate personal protective equipment, including
gloves and eye protection. - Spills or leaks should be cleaned up promptly using appropriate methods, and
contaminated materials should be disposed of
in accordance with local regulations.